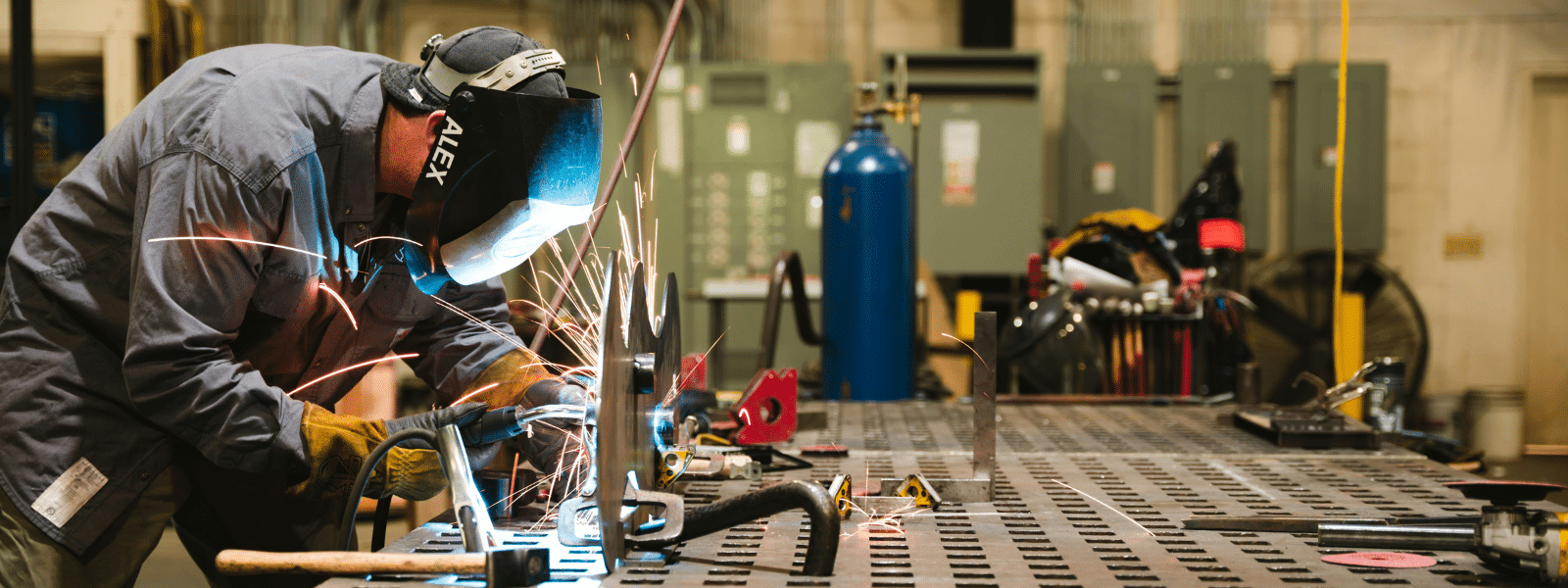
Proper chemical treatment is essential for disinfecting water and preventing buildup in boilers. But what other benefits are there? In this blog...
Blog

CHEMICAL INDUSTRY NEWS
Chemical Chat – Discover What’s New!
How to Use Industrial Cleaning Chemicals: Best Practices
There are thousands of cleaning solutions on the market. But how do you know which one is right for your business? In this blog post, we’re going to...
Are Denatured Alcohol and Isopropyl Alcohol the same?
Denatured alcohol and isopropyl alcohol are commonly used alcohols that each boast a wide variety of applications. As it turns out, they are not the...
Parts Washer Solvent: Optimal Cleaning for Industrial Components
In industrial cleaning, parts washer solvents have become essential for achieving effective and meticulous cleaning of...
How to Use Industrial Cleaning Chemicals: Best Practices
There are thousands of cleaning solutions on the market. But how do you know which one is right for your business? In...
Company News

Managed Services
Discover the Latest in Safe and Sustainable Chemical Solutions
Stay informed with Ecolink’s blog! Subscribe now
Chemical Management Information
Stay updated with us
Sign Up for the Latest Updates
Stay informed about chemical supply chain disruptions and emerging innovations to keep your business at the forefront of efficiency and innovation. Uncover new ways to make your business practices more sustainable by incorporating safer products into your cleaning lineup.