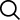Choosing the right eco-friendly degreaser for your business can be tough—from finding suppliers to combing through page after page of products, it’s...
Blog

CHEMICAL INDUSTRY NEWS
Chemical Chat – Discover What’s New!
Cleaning Electrical Contacts: 5 Tips for Effective Maintenance
Electrical contacts are important circuit components found in machines and electrical equipment that are made from conductive materials. When two...
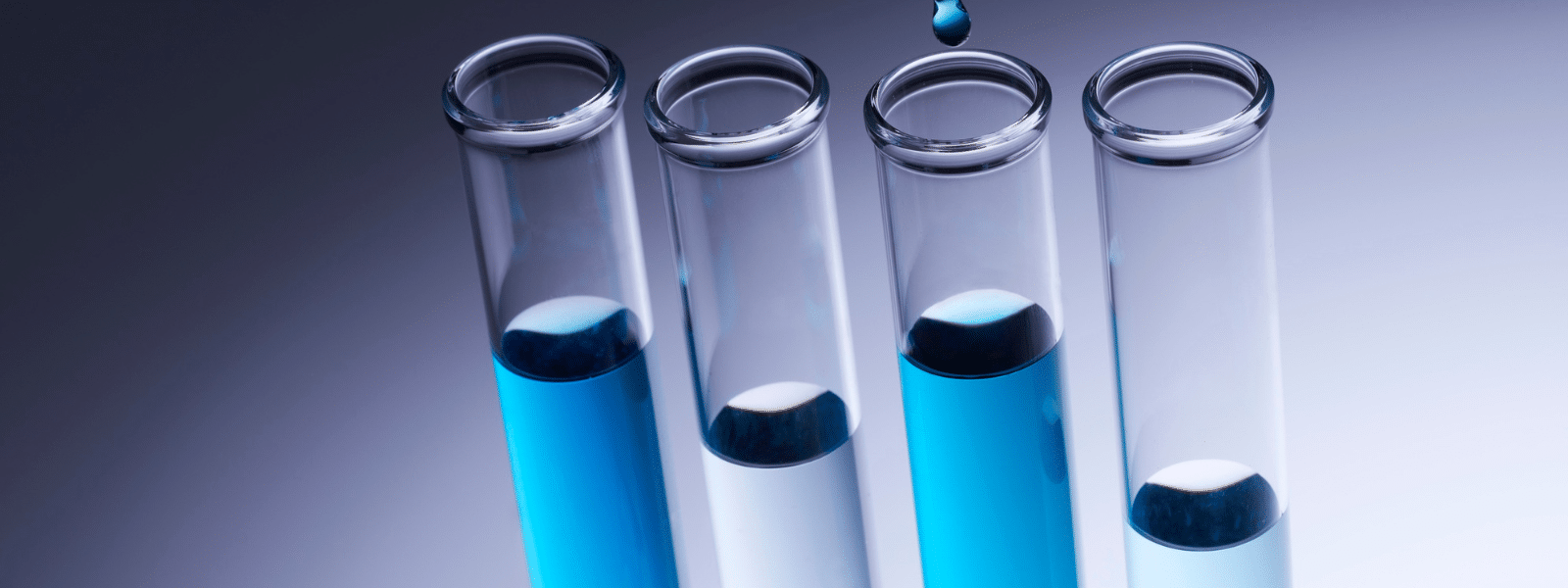
Top 3 Electrical Cleaning Solvents to Extend Equipment Life
Manufacturers utilize various electrical equipment for industrial tasks daily. Electrical equipment can aid in production time and complete tasks...
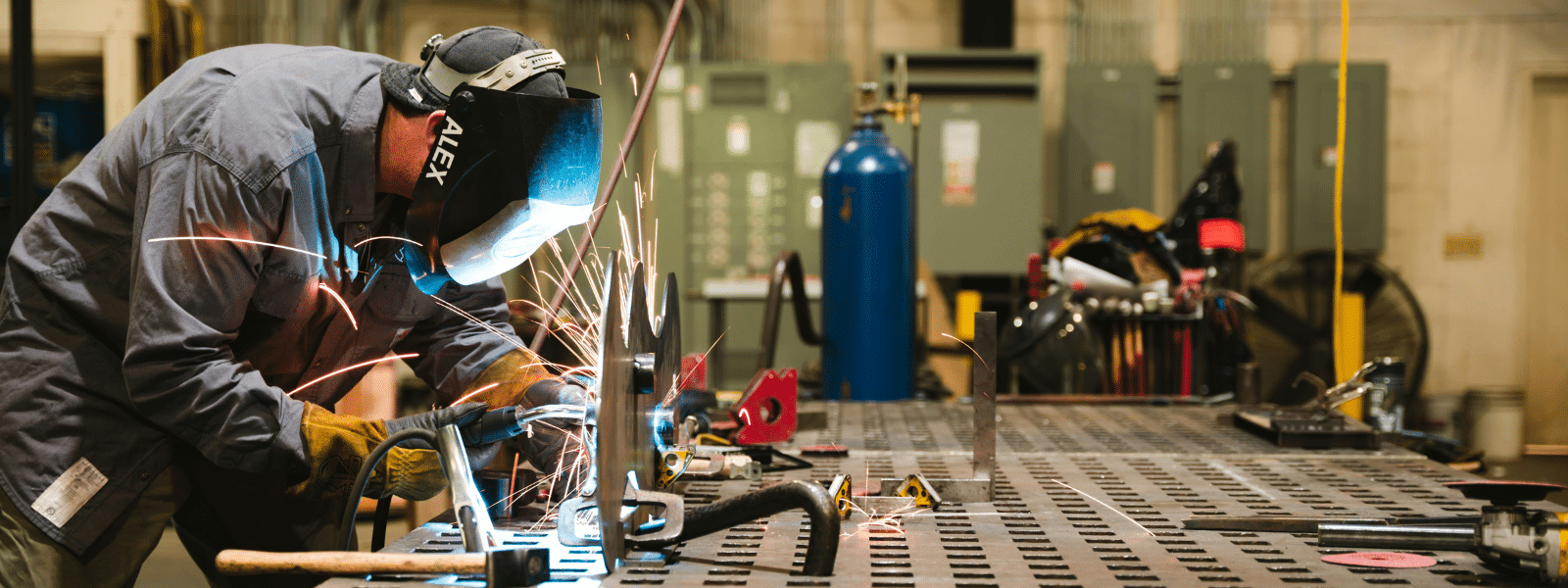
Mechanical Cleaning: Essential Techniques for Industrial Maintenance
In industrial maintenance, cleaning stands out as a fundamental process for ensuring optimal equipment performance and...
Cleaning Electrical Contacts: 5 Tips for Effective Maintenance
Electrical contacts are important circuit components found in machines and electrical equipment that are made from...
Company News

Managed Services
Discover the Latest in Safe and Sustainable Chemical Solutions
Stay informed with Ecolink’s blog! Subscribe now
Chemical Management Information
Stay updated with us
Sign Up for the Latest Updates
Stay informed about chemical supply chain disruptions and emerging innovations to keep your business at the forefront of efficiency and innovation. Uncover new ways to make your business practices more sustainable by incorporating safer products into your cleaning lineup.